Abstract
Introduction: Stromal Antigen 2 (STAG2), located on chromosome Xq25, is the most mutated cohesin complex (CC) gene in myeloid neoplasms (MN), mostly MDS and AML. Somatic STAG2 mutations (STAG2m) are described as "secondary-type", specific for secondary AML (sAML). With mixed existing data regarding response to therapy and its impact on survival, we did a single institutional retrospective study on impact of STAG2 mutations in MN.
Methods: After IRB approval, we retrospectively screened 3635 patients (pts) who had next generation sequencing (NGS) performed at Mayo Clinic between 2018-2021. Pts harboring STAG2m were identified (n=91/3635 [2.5%]), and their records reviewed for clinical information. Pts were included at time of in-house NGS. BlueSky Software V7.40 was used for statistical analysis.
Results:
Characteristics: Majority of pts were males (78%), with median age of 72 years. Diagnosis included MDS (55%), AML (29%), MDS/MPN (10%) and MPN (3%). Two CCUS and 1 aplastic anemia (AA) case were included. Therapy-related MN (tMN) was identified in 15%. Of MDS cases, 72% were MDS-EB and 46% were IPSS-R high/very high risk. Of AML cases, 38% were sAML. An abnormal karyotype was found in 28 pts (31%), with trisomy 8 being the most common. MN diagnosis was made in 47 pts (52%) prior to NGS.
STAG2m: Median variant allele frequency (mVAF) was 50% (range 5-100%) and was higher in males (p<.001). VAF did not differ between diagnostic groups, MDS subtypes/risk groups or de novo (dn) and tMN. There was no correlation between VAF and blast count. STAG2m were nonsense (54%), frameshift (36%) and splice site (10%). The STAG domain had 6 (p.Arg259) and 4 (p.Arg216) nonsense mutations.
Co-mutations: Median number of co-mutations was 3 (range 0-6) and did not correlate with MN classification. ASXL1 (65%), TET2 (36%), SRSF2 (36%), RUNX1 (30%) and BCOR (20%) were the most common co-mutations. High risk MDS pts had more BCORm (p=.01) and RUNX1m (p=.2) than lower risk pts. None of the co-mutation predicted progression to AML. Co-mutational pattern did not differ in dnAML vs sAML and dnMN vs tMN. Six pts had isolated STAG2m and were clinically like co-mutated pts.
Therapy outcome and AML progression: Therapy was given to 63 pts (69%) including 30, 16 and 6 who received HMA, HMA + venetoclax (VEN) and intensive chemotherapy (IC), respectively. Hematopoietic stem cell transplantation (HSCT) was done in 25 pts. Response rate was 46%, 63% and 83% for HMA, HMA+VEN and IC, respectively. There was no difference in response rate based on therapy regimen. Median overall survival (mOS) did not differ between HMA responders and non-responders. Relapse occurred in 54% of AML and 19% of MDS pts. Therapy regimen, cytogenetics and VAF did not impact relapse. Progression to AML occurred in 18% of MDS and 22% of MDS/MPN pts. Higher risk MDS were more likely to progress than lower risk (p=.04) and median number of co-mutations was 4.
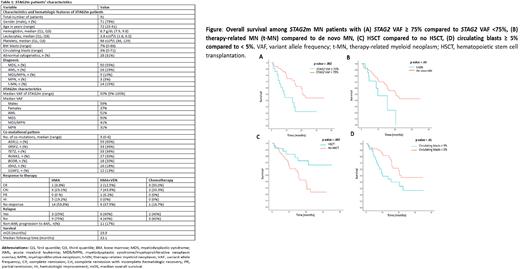
Survival and molecular dynamics: mOS among 88 pts (excluding CCUS and AA) was 19.9 months. There was no difference in survival based on gender (p=.2), cytogenetics (p=.08), MN classification (p=.3), MDS risk grouping (p=.6) or between dnAML vs sAML (p=.2). mOS was lower in tMN compared to dnMN (9.9 vs 20.5 months, p=.03), STAG2m VAF≥ 75% compared to <75% (8.2 vs 21.4 months, p=.002), and circulating blasts ≥5% compared to <5% (10.1 vs 21.4 months, p=.01). HSCT improved mOS (not reached vs 15.6 months, p=.007). All remained significant on multivariate analysis (HR=2.6, p= .03; HR=2.6, p= .005; HR=2.9, p= .002 and HR=.3, p= .008, respectively). Of 91 pts, 31 had subsequent NGS. STAG2m persisted in 16 (52%), and the number and pattern of co-mutations did not change. mOS was lower among those with persistent STAG2m (p=.03).
Conclusion:STAG2m was most common in elderly males and MDS. It was associated with high risk MDS and increasing genetic instability on AML progression. Isolated STAG2m were uncommon while common co-mutations were ASXL1, TET2, SRSF2, RUNX1 and BCOR. This implies a role of STAG2m in MN progression. STAG2m independently predicted poor prognosis, regardless of MN classification, especially in tMN and with circulating blasts ≥5%. HSCT had a favorable impact on STAG2m pts. Our study is limited by the small sample size and retrospective nature.
Disclosures
He:Kura Oncology, Inc: Consultancy. Begna:ImmunoGen: Research Funding; Novartis: Honoraria. Mangaonkar:Bristol Myers Squibb: Research Funding. Patnaik:Kura Oncology, Stemline Therapeutics: Research Funding. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board. Shah:Marker Therapeutics: Research Funding; Astellas: Research Funding; Celgene: Research Funding. Foran:Novartis, Servier, Pfizer, BMS, Taiho: Other: Formal Advisory Activities; AbbVie, Actinium, Aptose, Astex, H3Biosciences, Kura Oncology, Trillium, Xencor: Research Funding. Al-Kali:Astex: Other: research support to institution.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal